Patrick Collombat, Inserm Research Director and head of the Avenir team at the Institut de Biologie Valrose in Nice, has published new results concerning Type I diabetes. Researchers show that, in mice, the pancreas contains cells capable of being converted into insulin-producing β cells, something that can be done at any age. They also demonstrate that all pancreatic β cells can be regenerated several times and that chemically-induced diabetes in mice can thus be “treated” repeatedly. The challenge for the researchers is now to show that these procedures can be applied to humans.
This work is published online in the Developmental Cell journal dated 27 June 2013.
Type I diabetes, characterised by the selective loss of pancreatic, insulin-producing β cells, is a condition that affects more than 30 million people worldwide. Despite current treatments, type I diabetic patients have a life expectancy that is reduced by five to eight years. It is in this context that the Avenir “Diabetes Genetics” team have been working to develop new approaches designed to regenerate these cells.
In 2009, researchers at the Valrose Biology Institute (Inserm/University Nice Sophia Antipolis) managed to convert glucagon-producing α cells into β cells in young mice. Today, thanks to the use of transgenic mice, they report the mechanisms resulting in this exchange of cell identity. Specifically, they show that pancreatic ductal cells can be continuously mobilised and literally transformed into α and subsequently into β cells, a process that works at any age. Such transformation is obtained through the forced activation of the Pax4 gene in the α cells of the pancreas. The resulting cascade of events causes the generation of brand-new β cells, thanks to the reactivation of development genes. Throughout this process, α cells are regenerated and gradually adopt the profile of β cells. This means that the pancreas has a virtually inexhaustible source of cells capable of replacing the β cells.
β cell regeneration in the pancreas
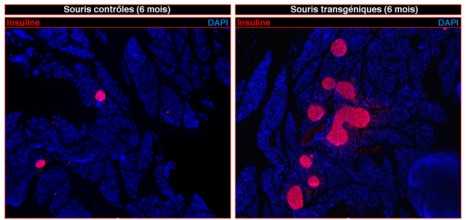
© Patrick Collombat / Inserm
Left: pancreas of control mice (non-diabetic)
Right: pancreas of transgenic mice demonstrating massive regeneration of insulin-producing β cells (coloured pink) following chemical induction of diabetes.
By artificially inducing type I diabetes in mice, “we also show that all the pancreatic β cells can be regenerated at least three times using this mechanism. Diabetes, induced in this way, in the mouse, can be literally “treated” multiple times thanks to the new stock of functional, insulin-producing β cells”
explains Patrick Collombat, Inserm research director and principal author of the study.
These promising results obtained in the mouse suggest that the pancreas contains cells that can regenerate several times those β cells lost in type I diabetics.
“We are currently working on the possibility of inducing such regeneration by using pharmacological molecules. Thanks to this new data, we shall be concentrating in future years on determining whether these processes can also be made to work in humans, a real challenge in offering better treatments for type I diabetic patients”, he concludes.